Erupting Pumpkins DIY Science Experiment
Erupting Pumpkins DIY Science Experiment

Halloween is over, and now you're left with a two-day-old jack-o'-lantern on your porch. Why not turn it into a science experiment?? This DIY science experiment, courtesy of the blog "Growing a Jeweled Rose," just gushes with fun!
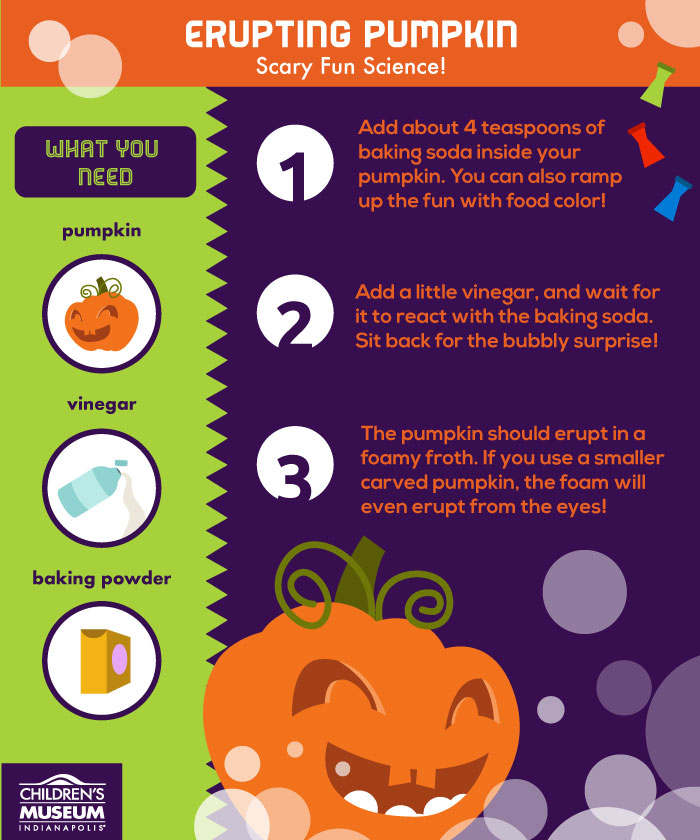
 Materials
Materials

- Carved pumpkin
- Baking soda
- Vinegar
- Food coloring
 Process
Process
- Fill your pumpkins with a bit baking soda (about 4 tablespoons per pumpkin).
- Add a few drops of food coloring.
- Add vinegar...and let the eruptions begin!
 Results
Results

Carbonic acid is unstable, and it immediately falls apart into carbon dioxide and water. The bubbles you see from the reaction come from the carbon dioxide escaping the solution that is left. Carbon dioxide is heavier than air, so it flows almost like water when it overflows the container. It's a gas that you exhale (though in small amounts), because it's a product of the reactions that keep your body going.

What's left is a dilute solution of sodium acetate in water.
Buy Haunted House Tickets
Buy tickets to the nation's longest running continually-operated haunted house. You'll have a a fa-BOO-lous time.









